Explain Why Are There Differences Between Logp Logp and Logd
Log D is a distribution coefficient widely used to measure the lipophilicity of ionizable compounds where the partition is a function of the pH. For example expand log₂ 3a.
Measuring Pkas Logp And Solubility By Automated Titration
Method one is based on 94 atomic contributions evaluated from 830 molecules by least squares analysis.

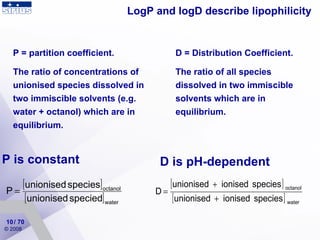
. Log p partition coefficient for n-octanolwater - three fragmentation methods are used to predict the log p values. When logP 0 the compound is equally partitioned between the lipid and aqueous phases. Probably the next two reasons can lead to the difference between the experimental and the predicted logD curves.
Early measurements of log P showed that broadly speaking π values were additive but measurements became necessary for different parent systems such as phenols and anilines since there were systematic differences between them due principally to electronic effects. The natural conclusion is that log P and log D are the same for un-ionizable. So log base 100 of 1 is going to be equal to 0.
They are just two different methods for calculating logP. Weve got the study and writing resources you need for your assignments. V KS.
1I want to simulate a PIN photodiode under reverse voltage and i region is active layer absorb 2I used the impact ionization model. Up to 10 cash back There is no pK a between two tautomers with the same formal charge because they have the same number of protons so their relative probability is independent of pH. According to the equation log D of a compound is always smaller than its log P value and the difference is determined by the fraction of ionized compounds at a given pH.
Well anything that a 0 power is equal to 1. 3When the negative voltage increases to 50Vthe. Lipophilicity _____ increases or decreases as molecules become more ionized.
275ff When one of the solvents is water and the other is a non-polar solvent then the log P value is a measure of lipophilicity or hydrophobicity. EE-MCC logP values have a mean error of 18 logP units versus experiment and a standard error of the mean of 10 logP units for three separate calculations. These properties apply for any values of and for which each logarithm is defined which is.
I used 741 compounds experimental logP data and the result is like below. To find the constants a and b we can substitute two widely-spaced points which lie on the line into the appropriate equationThis gives two equations for the two unknowns a and b. Unlike log P which is pH-independent log D changes with pH as the fraction of each species shifts.
These errors are primarily due to getting sufficiently converged energies to give accurate differences of large numbers particularly for the large-molecule solvent octanol. Lipophilicity is an important physicochemical parameter that contributes to the absorption distribution metabolism excretion and toxicity of a drug. The area logP is large the accuracy is very high.
This in turn affects the solubility and permeability of a drug and contributes to its potency and selectivity. LogD the distribution constant is a better descriptor of the lipophilicity of a molecule. Un-ionized ionized ionized at some other positionall species go into the equation.
Experimental RT values were regressed on logP estimates from all three sources for the training set chemicals n 78. Log D is thus pH dependent hence the one must specify the pH at which the log D was measured. The logPi value seems to be correct in our logD model.
A straight line on a semilog graph of y versus x represents an exponential function of the form y a e b x. The effect of the support electrolyte on the ionizedunionized molecule is not considered correctly 2. So in this case x is equal to 0.
OPERA model predictions of logP referred to as OPERA logP are based on k-nearest neighbors kNN classification of molecular descriptors. It will also explain why the same dose may cause a therapeutic effect by one. LogP 1 means there is a.
Intro to logarithm properties. Jones MR Brooks BR 2020 Quantum chemical predictions of water-octanol partition coefficients applied to the. The pH-independent free energy difference between.
Commonly expressed as LogP or LogD Permeability increases as K or logD increases. Log base anything of 1 is going to be equal to 0 because anything to the 0 power and were not talking about 0 here. Learn about the properties of logarithms and how to use them to rewrite logarithmic expressions.
The logPi. This method works with a standard deviation of 0. A straight line on a log-log graph of y versus x represents a power law function of the form y a x b.
The partition coefficient abbreviated P is defined as a particular ratio of the concentrations of a solute between the two solvents a biphase of liquid phases specifically for un-ionized solutes and the logarithm of the ratio is thus log P. For a nonionizable compound its log D equals to log P. But the area logPwhy I can estimate logP with this simple group additivity method.
A positive value for logP denotes a higher concentration in the lipid phase ie the compound is more lipophilic. 6 The disadvantage of a substituent constant approach is that any models constructed using the. I found the answer.
This can be determined in a similar manner to LogP but instead of using water the aqueous phase is adjusted to a specific pH using a buffer. - different equations for low and high concentrations. Log D describes the distribution of all forms of the compound at a specific pH.
It is often observed that drugs that are in the early stages of development have. OPERA logP is distinct from OPERA-RT the model used to predict RT. Lipophilicity LogP LogD Oral absorption cell membrane db penetration distribution Chemical stability Chemical integrity in body fluids tissues and oral absorption Metabolic clearance Bioavailability and clearance CYP450 inhibition Metabolism and.

Comparative Linear Relationship Between Log P Reference And The Download Scientific Diagram

Logp And Logd Calculations Chemaxon Docs

Comparison Of Logp And Logd Values Between Existing And Potential Download Table

Comparison Of Logp And Logd Values Between Existing And Potential Download Table
No comments for "Explain Why Are There Differences Between Logp Logp and Logd"
Post a Comment